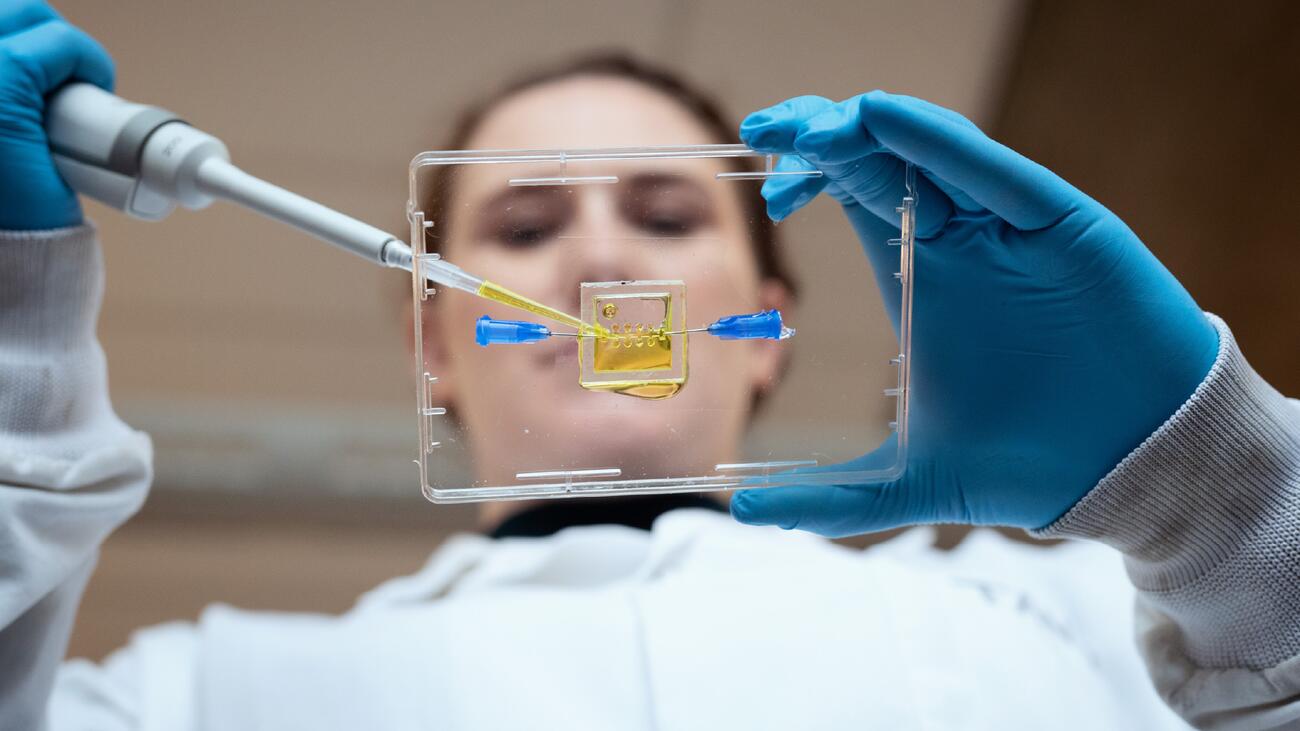
Tanya Bennet, a PhD student in Biomedical Engineering, is pipetting hydrogels that serve as scaffolds for neural cells, in the development of an in vitro model of the injured spinal cord. Photo: Martin Dee
Brainwaves
Serious disorders affecting the brain and nervous system can be complex to treat. UBC researchers are exploring some promising new avenues.
SPINAL CORD INJURY
A multidisciplinary research team hopes to mend the gap
A car crash, a sporting collision, a fall from a ladder — incidents like these can change lives in an instant. If the spinal cord snaps, the resulting gap means nerve impulses from the brain can’t reach the body. That disruption can cause significant health problems including chronic pain, sexual dysfunction, loss of bladder or bowel control, and paralysis.
Spinal cord injury (SCI) also poses serious challenges for healthcare treatment. Invasive surgery and the use of solid materials for repairing the gap carry the risk of damaging any remaining nerve fibres and bodily functions.
An innovative approach to treatment offers hope. UBC’s multidisciplinary Mend the Gap team is developing a soft gel biomaterial that a machine-vision-equipped surgical robot can inject precisely into the point of injury to help nerve fibres regrow. In contrast to previous treatments, the biomaterials are compatible with bodily systems and structures, says research lead Dr. John Madden, a UBC electrical and computer engineering professor. The gels can also contain medication to modify scar tissue (which can complicate repair) as well as revive nerve fibres.
“The soft gel that our team plans to use contains tiny magnetic rods that are aligned using an external magnet, creating guide rails that support the nerve fibres to grow in the right direction, eventually crossing the gap,” Madden says.
UBC surgery and zoology professor Dr. Wolfram Tetzlaff points out that because invasive surgery is minimized, recovery times could be faster and the potential for damage is reduced. Beyond SCI, the researchers hope to find ways of applying this treatment to other chronic injuries. “A soft gel can be moulded into the shapes of the many different lesions seen in the body, and thus provide personalized treatment,” says Tetzlaff.
Due to the complexity of the nervous system, a cure for SCI is not currently the goal. But the team hopes the project will lead to increased motor function, a longer life span, and a better quality of life.
BRAIN CANCER
Tiny receptors could be a key new target for treatments
Brain cancer. It’s the diagnosis no one wants to hear. Patients with high-grade gliomas, or tumours in the brain and spinal cord, have an average life expectancy of 12 to 16 months. Not only do tumours in the brain spread more aggressively than in other tissues, but they are also resistant to chemotherapy and have a high probability of recurring after surgical removal.
Two UBC Okanagan researchers are working to better understand the development and rapid growth of cancerous cells in the brain. Sessional lecturer Dr. Mitra Tabatabaee and Dr. Fred Menard, a professor of chemistry, biochemistry and molecular biology, examined astrocytoma, a highly fatal cancer with no effective treatment that begins in astrocytes – cells that support nerve cells.
They analyzed the potential role of an imbalance of glutamate – a neurotransmitter that stimulates nerve cells – in astrocytoma progression. Their findings suggest that several receptors not previously considered in brain cancer research might be crucial to cancer’s growth.
Star-shaped astrocytes extend tentacle-like cellular projections to communicate with neighbouring cells. When astrocytes become cancerous, these projections become longer, and their networks more complex, invading different areas of the brain. How far they extend is implicated in the cancer’s resistance to treatment, as any extra-long cell projections left behind during surgery can grow back.
A suspected cause of this uncontrolled growth is elevated levels of glutamate. When astrocytes sense glutamate, the concentration of calcium rises inside the cell. Since calcium is also necessary for growing cellular projections, the glutamate receptors that affect the calcium inside astrocytes are prime suspects for the abnormal growth of astrocytoma cells.
Tabatabaee and Menard identified a glutamate receptor and two other molecular contributors crucial in extending the projections of cancerous cells. With further study, researchers believe that these overlooked receptors can serve as targets for designing more effective chemotherapies and open up new avenues to halt the progression of this aggressive and often fatal cancer.

ALZHEIMER’S
Lab-grown mini-brains reveal processes of brain degeneration
Two UBC researchers are using stem cell technology, 3D bioprinting, and next-generation neuroimaging to grow and analyze tissue models of patient’s brains. Their aim is to better understand the mechanisms of Alzheimer’s disease and inform new options for treatment.
Dr. Haakon Nygaard (Fipke Professor in Alzheimer’s Research and director of the UBC Hospital Clinic for Alzheimer Disease and Related Disorders) and Dr. Brian MacVicar (UBC psychiatry professor and Canada Research Chair in Neuroscience) are collaborating on a pioneering project to determine the role that oxidative stress plays in the death of brain nerve cells. “Understanding the specific processes that trigger oxidative stress is key to developing successful targeted therapies for people with Alzheimer’s,” explains MacVicar.
Using stem cells taken from the blood of Alzheimer’s patients and a 3D bioprinter, the researchers are growing tissue models of the patients’ brains in petri dishes. Each model, Nygaard clarifies, is “a 3D ball of tissue that incorporates the major cell types of the brain” but, unlike the brain, is “not a complex, multi-layered structure.” These 100 “neurospheres” enable researchers to closely follow and gain insights into the basic cellular mechanisms that cause the brain to degenerate into Alzheimer’s. MacVicar has developed techniques using state-of-the-art neuro-imaging to help them visualize these processes.
They have nearly completed a three-dimensional model that incorporates the main cell types of the brain. This model, says Nygaard, will help them find answers to long unanswered questions, such as how different cell types interact. “Once we have modelled the progression of Alzheimer’s in many different patients, the data should yield valuable new insights into how, ultimately, we can reduce oxidative stress and protect brain nerve cells.”
Both Nygaard and MacVicar are optimistic that their work could lead to the development of more effective diagnosis and intervention, and maybe even prevention.
MULTIPLE SCLEROSIS
New research network is developing next-generation cell-based therapies
Multiple sclerosis (MS) is an unpredictable and incurable disease where the immune system mistakenly attacks and destroys nerve cells in the brain and spine. Symptoms can vary greatly, from dizziness and muscle spasms to loss of vision, speech, or movement.
Thanks to an unprecedented $33.8 million donation from an anonymous donor, UBC and partners are forming the MS Research Network headquartered at the Djavad Mowafaghian Centre for Brain Health, which will use the latest in cell and gene engineering to develop, manufacture, and test next-generation cell-based therapies. Researchers spanning multiple disciplines are collaborating to tackle different aspects of the disease, with the aim of slowing the progression of MS by controlling symptoms and even reversing the damage it causes to the nervous system.
Surgery and biomedical engineering professor Dr. Megan Levings is leading research that could make immunosuppressant treatments more effective for more patients. These treatments can leave patients vulnerable to infection, but her research team has proven that T cells, which control the body’s response to healthy tissue, can be trained to recognize and accept specific tissues that a faulty immune system would attack. The goal is to control inflammatory responses that contribute to damage of the nervous system.
Meanwhile, medical genetics professor and neurobiologist Dr. Freda Miller is leading an exploration of how stem cells could be used to repair damage caused by MS, which destroys myelin, the fatty protective sheath covering nerve cells in the brain and spine. “The beauty is that the brain contains reserves of neural stem cells. With the right chemical prompts, they can be converted into cells that produce myelin, replacing the ones destroyed by MS,” says Miller.
The combination of the research by Dr. Miller’s team with Dr. Leaving’s immunotherapy could offer patients the possibility of recovering.
“In a perfect world, we figure out a way to regenerate the damaged areas, while our colleagues in immunology train the immune system to leave the new myelin alone,” Dr. Miller says. “It’s a one-two punch.”
PARKINSON’S
Machine Learning for better disease monitoring and treatment
A person with Parkinson’s disease will typically be monitored just once or twice annually by their physician, but a clinic visit is often a poor reflection of how people are doing in the comfort of their own homes. Dr. Martin McKeown, John Nichol Chair in Parkinson’s Research, says that’s because symptoms can fluctuate due to multiple factors. Researchers at the Djavad Mowafaghian Centre for Brain Health are working on new ways to unobtrusively monitor patients between visits.
One approach is the deployment of privacy-compliant smart cameras to empower people to monitor their disease at home. The new cameras, coupled with analyses being developed by McKeown and colleagues, are powerful enough to analyze the data (without requiring images to be stored or transferred) to help inform treatment decisions. Another initiative involves an app that can remotely measure and score 12 movements involving fingers, arms or feet – an assessment that previously required a clinic visit.
When Parkinson’s medication wears off, it can be very uncomfortable, but taking too much medication to prevent this results in other problems. Predicting wearing-off symptoms an hour before they happen would allow enough time to take new medication and have it take effect. Researchers are thus trying to ascertain if a wearable sensor can provide this information, which could eventually be used to guide dosage and timing.
McKeown and colleagues are also using various brain imaging techniques to determine the best ways to non-invasively modulate the brain (via an electrical current or ultrasound) to improve motor function.
Tying all these projects together is the requirement of analyzing huge amounts of data, all while ensuring that the privacy of individual patients is integral to the design. Data scientist and research associate Dr. Maryam Mirian oversees the analysis of these data using various AI and machine learning approaches.
Ultimately, machine learning techniques applied to “big data” will provide a more complete understanding of what is happening in the daily lives of patients, contributing to much-needed advances in treating this degenerative disorder.